In the MedTech industry, product traceability is not just a regulatory necessity – it is a key component in ensuring patient safety, maintaining quality standards, and ensuring regulatory compliance. A well-developed traceability system allows companies to monitor medical devices throughout their entire lifecycle, from design and production to distribution and use. This traceability is crucial not only for responding quickly to defects but also for ensuring that all regulatory requirements are met.
Traceability as a Tool for Quality Improvement and Defect Management
Effective product traceability is a crucial factor in handling product recalls. When defects are detected in devices, a robust tracking system enables faster and more targeted recalls. This is essential for minimizing risks to patients and ensuring that any defective products are quickly removed from the market. Without an effective tracking system, companies risk exposing patients to hazardous products, which can have serious consequences.
Traceability is key to complying with the strict requirements set by authorities such as the FDA (Food and Drug Administration). Regulations require thorough documentation and registration of medical devices. Without proper traceability, companies risk sanctions.
Quality Control and Process Improvement Through Traceability
Product traceability supports quality control by enabling thorough monitoring and documentation of all production processes. This makes it possible to identify and correct defects before the product reaches the market. Traceability also provides insight into which specific product batches are affected if a problem arises. This enables companies to conduct fast and efficiently targeted recalls, preventing further harm.
Elements of an Effective Traceability System
For a traceability system to be effective, it must include several key elements:
- Unique Device Identification (UDI):
Unique Device Identification (UDI) ensures that each unit can be accurately traced throughout its entire lifecycle. This is essential for ensuring proper documentation and tracking of products. - Comprehensive Documentation:
An effective traceability system provides insight into materials, processes, and the employees involved in the production of the devices. This documentation is necessary to maintain quality and compliance with regulatory requirements. - Technology Integration:
Technologies such as barcodes, RFID (Radio Frequency Identification), and blockchain can play an important role in strengthening traceability by enabling real-time monitoring and ensuring data integrity.
Nordcad’s Solutions for Enhanced Product Traceability
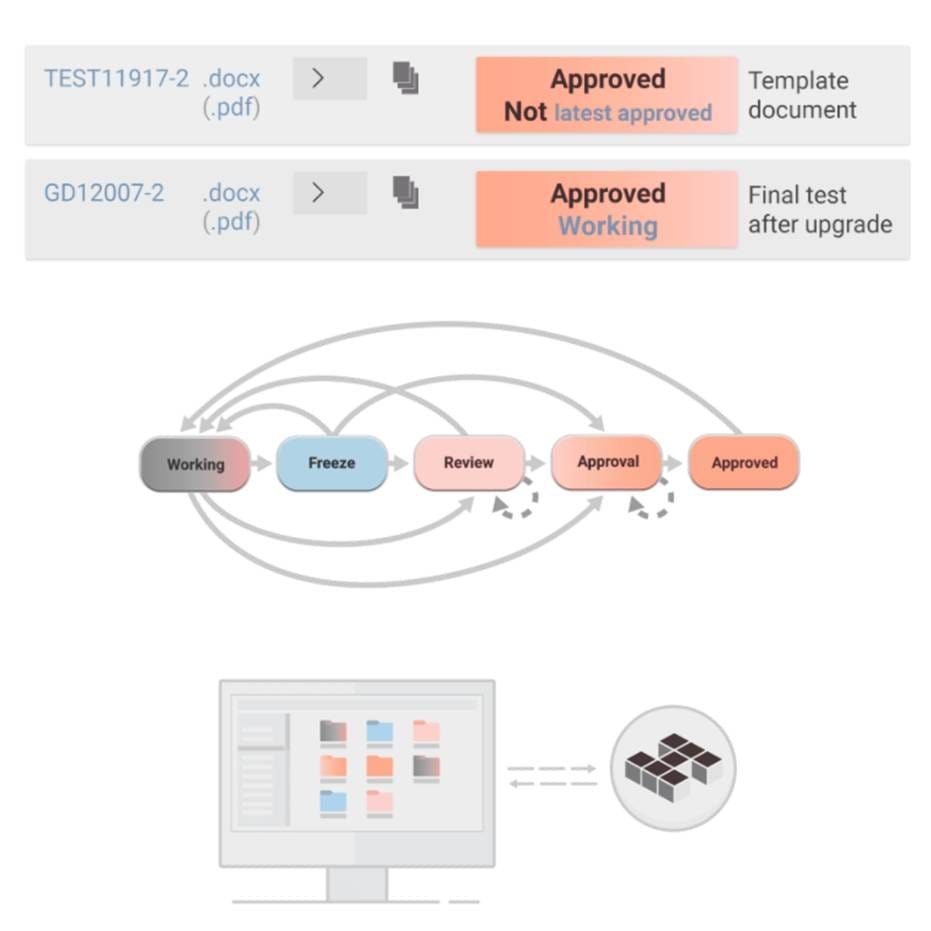
At Nordcad, we work closely with our partners to offer solutions that enhance traceability in the MedTech industry. Our digital document management system centralizes all product-related documentation and ensures easy access to data with high security.
This system allows companies to have full control over product information while protecting against data loss.
Our solutions also support process validation, helping companies ensure that their production complies with current legislation and quality requirements. By integrating real-time monitoring into the production process, deviations can be quickly detected and corrected, leading to a more efficient and safer production environment.